ISO 13485 Medical devices quality management systems
The world of medical science has seen an enormous amount of development aimed at saving the lives of countless individuals. Medical devices, which can be a hardware or a software used in treating human beings or in diagnosis of diseases, causes a drastic improvement towards the quality of life and treatment for patients. Surgically implanted devices such as artificial pacemaker, cochlear implants, drug-elating stents or implantable pills, etc., are exhibiting much higher success rate and need thus, posing a significant effect on patient safety and risk management in medical business. However, without a system in place which warrants proper safety and quality procedures during the production the same devices can pose a threat to human health.
Therefore, a quality management system is critical to ensure that the medical devices will be manufactured to specifications for safe intended use every time. For these very reasons, most national regulatory schemes require manufacturers and suppliers of medical devices to establish an internal quality management system that has been independently audited and verified. Organizations whose QMS hasn’t been verified usually end up being legally denied thereby, loosing revenue opportunities.
The primary goal of drafting ISO 13485: 1996 was to harmonize medical device regulatory requirements and some requirements of ISO 9001 that were deemed not appropriate as regulatory requirements for quality management systems.
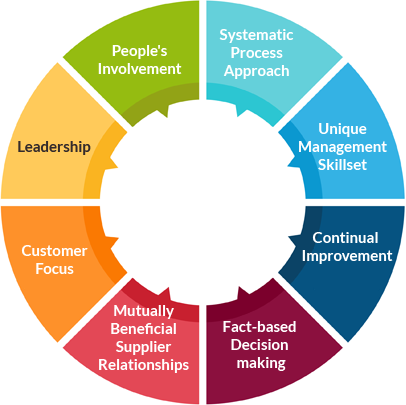
ISO 13485: 2016 is based on eight quality management principles which when fully adopted, have been proven to enhance organizational performance. They are,
The standard does not provide a direct solution to design quality. However, it helps to create a framework which enables the use of various solution models depending on the one hand on manufacturer’s size, the complexity of the products and the potential risks and on the other the quality targets set by manufacturers themselves with regard to both their own operations and the devices.It is a stand-alone QMS standard, derived from the internationally recognized and accepted ISO 9000 quality management standard series which is accepted as the basis for CE marking medical devices under European Directives.
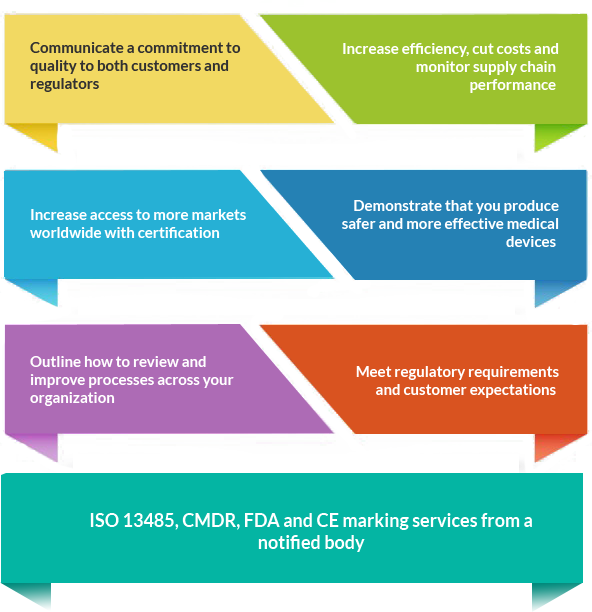
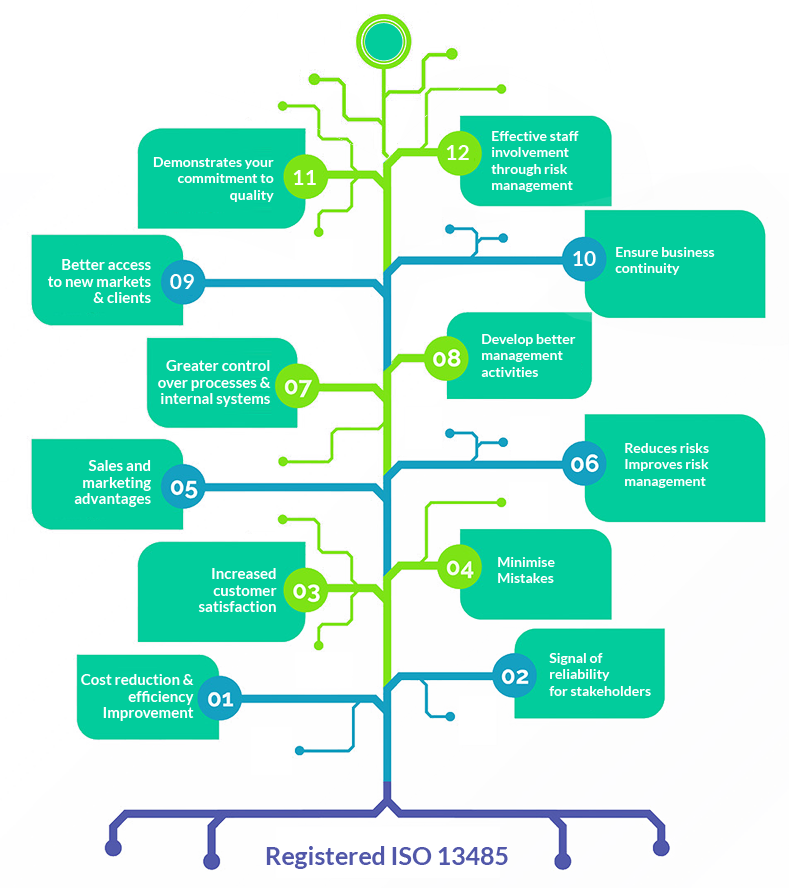
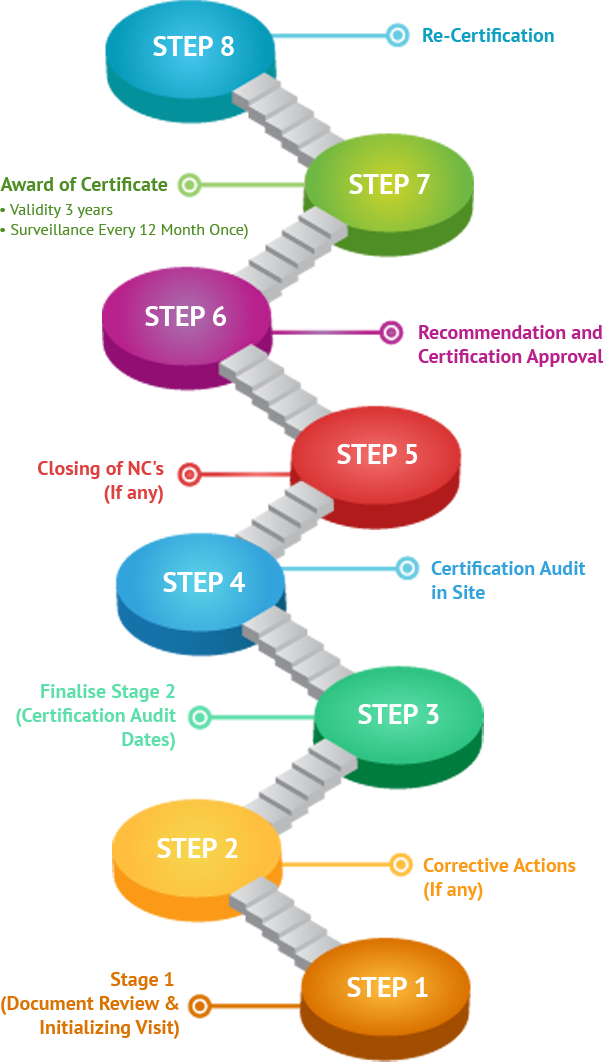
Whether you are looking to operate internationally or expand locally, ISO 13485 Certification can help you improve overall performance, eliminate uncertainty, and widen market opportunities. Companies with ISO 13485certification